Don't miss our holiday offer - up to 50% OFF!
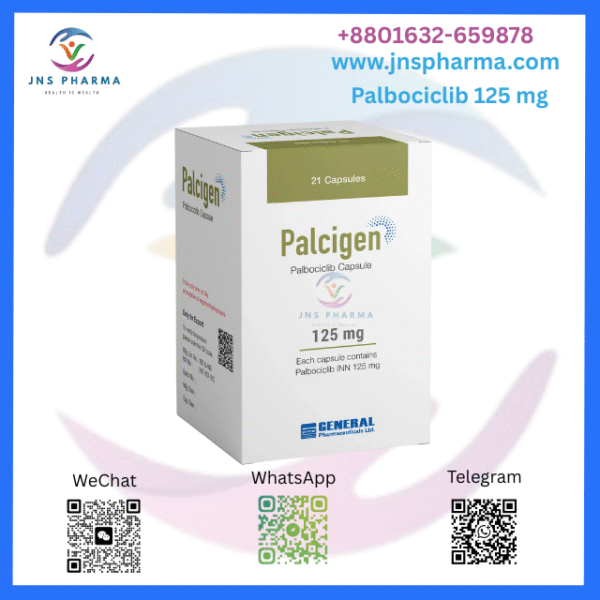
Palcigen 125 mg (Palbociclib)
Palbociclib is the active ingredient of the anticancer medication Palcigen 125 mg. It’s a drug that falls under the order of cyclin-dependent kinases (CDK) 4/6 impediments, and it’s most extensively used for the treatment of certain types of bone cancer. Palcigen inhibits the growth of cancer cells by snooping with certain proteins involved in cell division. It’s generally indicated for hormone receptor-positive (HR), mortal epidermal growth factor receptor 2-negative (HER2-) advanced or metastatic breast cancer, constantly in confluence with hormone treatments similar as letrozole or fulvestant.
It represents an important corner in the treatment of bone cancer, especially in those who have little response to the usual chemotherapy.
Composition and Strength
Brand Name Palcigen
general Name Palbociclib
Strength 125 mg
Lozenge Form Oral capsules
Each capsule contains 125 mg of Palbociclib and inactive constituents that allow for capsule stability and immersion.
Mechanism of Action
Palbociclib widely inhibits CDK4 and CDK6, enzymes that are necessary for cell cycle progression. typically, CDK4/ 6, bound to cyclin D, drive cells from the G1 phase into the S phase of the cell cycle, easing DNA replication and cell division. In most cancers, these conditioning are hyperactivated and lead to unbridled growth of cancer.
By inhibiting CDK4/ 6 exertion, Palcigen causes cell cycle arrest at the G1 phase to inhibit the proliferation of cancer cells. Interestingly, this action is more specific for cancer cells than normal healthy cells, making it a targeted remedy with smaller side effects than conventional chemotherapy.
Indications
The following conditions can be treated with Palcigen 125 mg:
Postmenopausal women with HR-positive, HER2-negative advanced or metastatic bone cancer in combination with aromatase impediments like letrozole as original endocrine- grounded remedy.
individualities with complaint progression following endocrine treatment, where it’s administered with fulvestrant.
It isn’t useful for HER2-positive or triadic-negative bone cancers, since those are treated else with other targeted curatives.
Dosage and Administration
It is advised to take 125 mg orally once a day.
Treatment Cycle handed on a 21 days on/ 7 days off schedule (28- day cycle).
With or Without Food. For better immersion, it’s to be taken with food.
Missed Cure If a cure has been missed or heaved, the case should n’t take an redundant cure but the coming due cure.
Cure adaptation may be needed according to case forbearance and toxin. The drug is continued until inferior toxin or complaint progression.
Combination Therapy
Palcigen is infrequently used as a single agent. rather, it’s added to the hormonal treatments, which enhances its efficacity
With Letrozole (Aromatase asset) given as first- line remedy for postmenopausal women.
With Fulvestrant (Estrogen receptor antagonist) in cases with former endocrine treatment.
This binary medium operates against bone cancer in two mechanisms hormonal repression and cell cycle inhibition.
Side Effects
As with all specifics, Palcigen can be associated with side effects, although not inescapably in all individualities. Generally being and clinically significant side effects are:
Veritably Common Side Effects
Low white blood cell counts, or neutropenia, increase the risk of infection.
Fatigue and weakness
Nausea and mild gastrointestinal worried
Stomatitis (mouth ulcers)
Hair thinning or loss of hair
Less Common but Serious Side Effects
Infections due to reduced impunity caused by reduced white blood cells.
Anemia and thrombocytopenia: Fatigue or bleeding risks may result from decreased red blood cells and platelets.
Liver function abnormalities
Pulmonary toxin (rare cases of interstitial lung complaint or pneumonitis)
Routine blood draws are advised for cases to cover blood counts and liver function while witnessing treatment.
Warnings and Precautions
Monitoring of Blood Count Neutropenia is a diurnal cure- limiting toxin, so routine complete blood counts( CBC) are important.
threat of Infection, Fever, chills, or any infection- suchlike symptoms should be informed to the doctor incontinently.
Gestation and Lactation: Palbociclib is dangerous to a developing fetus; it shouldn’t be used during gestation or lactation. Contraception should be effective both during remedy and after conclusion of the medicine for a period of time.
Impairment of Liver and feathers: Dosage adaptations are attainable.
Medicine relations Strong CYP3A impediments (e.g., ketoconazole) or corrupters (e.g., rifampin) may affect medicine metabolism and should be avoided.
Storage and Handling
Store at room temperature (15 °C to 30 °C).
Keep down from humidity and sun.
Palpociclib should be taken whole, not crushed or masticated.
Keep out of children’s reach.
Clinical Benefits
Palbociclib has been shown in trials to ameliorate progression-free survival (PFS) far better than hormonal remedy alone. Donors of palcigen experience frequently longer complaint control and bettered quality of life.
For illustration, the PALOMA- 2 and PALOMA- 3 trials demonstrated that Palbociclib plus endocrine remedy nearly doubled progression-free survival in HR/HER2-bone cancer cases compared to that for endocrine remedy alone.
Conclusion
Palcigen 125 mg (Palbociclib) is an innovative oral targeted remedy for HR-positive, HER2-negative advanced bone cancer. Through the inhibition of CDK4/ 6, it effectively inhibits cancer cell growth without exposing cases to the worst of chemotherapy. Though neutropenia and fatigue are the common side effects, proper monitoring and medical care will enable these pitfalls to be managed.
As part of a combination remedy with letrozole or fulvestrant, Palcigen has entered the mainstream as a standard form of treatment, significantly perfecting clinical benefits and offering new stopgap for cases who survive advanced bone cancer.



Reviews
There are no reviews yet.