Don't miss our holiday offer - up to 50% OFF!
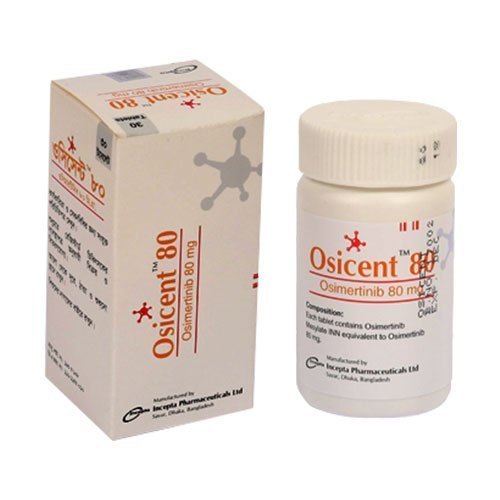
Osicent 80 mg (Osimertinib)
Third-generation EGFR tyrosine kinase inhibitor (TKI) Osimertinib is the active ingredient of Osicent 80 mg. It is primarily used to treat patients with specific EGFR gene mutations who have non-small cell lung cancer (NSCLC). Osimertinib has revolutionized the operation of advanced lung cancer due to its medium- grounded action, efficacity in complaint resistance, and good tolerability.
Mechanism of Action
Osimertinib widely inhibits mutant EGFR, including T790M mutation, an arising resistance mutation in cancer cases who have been preliminarily treated with first- or alternate- generation EGFR TKIs similar as erlotinib or gefitinib. unrecoverable mutant EGFR list blocks its signaling, thereby precluding cancer cell growth and survival. Osimertinib has lower exertion against wild- type EGFR, reducing side goods similar as skin rash and diarrhea that are out- target in nature.
Indications
Osicent 80 mg (Osimertinib) is indicated:
Adult patients with stage III non-small cell lung cancer (NSCLC) who had EGFR exon 19 elisions or exon 21 (L858R) genetic mutations were treated first.
Treatment of metastatic EGFR T790M mutation-positive NSCLC following complaint progression on or after EGFR TKI remedy.
Adjuvant treatment in EGFR mutation-positive NSCLC following resection of the excrescence to reduce the threat of cancer rush.
Dosage and Administration
Recommended Cure The recommended grown-up cure of Osicent is 80 mg orally formerly daily, with or without food.
The tablet should be consumed whole and not crushed or resolve.
Missed Cure If a cure is missed, it should be taken as soon as possible except for lower than 12 hours before the coming bone.
Duration of Treatment is to be continued until complaint progression or inferior toxin.
Pharmacokinetics
Immersion. It’s well absorbed orally and reaches peak tube attention in 6 hours.
Distribution: It has a large volume of distribution, indicating wide towel penetration, including into the central nervous system( CNS), thereby being effective against brain metastases.
Metabolism: It’s metabolized primarily by CYP3A4/ 5 enzymes.
Elimination: The half- life of the medicine is about 48 hours, with excretion largely through the feces.
Clinical Efficacy
Osimertinib has displayed sterling efficacity in numerous clinical trials
FLAURA Trial showed significantly bettered progression-free survival( PFS) and overall survival( zilches) as original treatment versus EGFR TKIs administered in a traditional fashion.
AURA3 Trial expressed advanced PFS in cases with T790M mutation-positive NSCLC who preliminarily progressed on EGFR TKI treatment.
It also shows central nervous system exertion, which is of critical significance since the maturity of NSCLC cases suffer brain metastases.
Side Effects
Even while Osicent 80 mg is usually well tolerated, some people experience negative side effects. Common side goods include
Diarrhea
Rash
Dry skin
Nail toxin
Fatigue
Rare but serious side effects include
Interstitial lung complaint/ pneumonitis
QT interval extension
Cardiomyopathy
Thrombocytopenia
Monitoring is advised during remedy in order to identify and manage the pitfalls beforehand.
Warnings and Precautions
Pulmonary toxin Discontinue incontinently in the event of a dubitation of interstitial lung complaint.
Cardiac goods Monitor cardiac and ECG status in cases with a history of heart complaint.
Medicine relations With strong corrupters of CYP3A( e.g., rifampin), concurrent use is to be avoided as they drop Osimertinib situations.
Gestation and Breastfeeding Fetal injury can be produced by Osimertinib. It must be avoided during gestation and breastfeeding.
Drug Interactions
Osimertinib can interact with
CYP3A impediments corrupters can change medicine tube attention.
Antacids or proton pump impediments. No dangerous commerce, but they should be taken at another time.
QT- dragging specifics. Because osimertinib itself has the ability to prolong the QT interval, caution is advised.
Use in Special Populations
No cure revision generally demanded, but monitoring should be guarded.
Renal Insufficiency Mild to moderate renal insufficiency has little impact on pharmacokinetics.
Hepatic Insufficiency Mild hepatic insufficiency has little impact, but caution should be used in severe hepatic insufficiency.
Benefits of Osicent (Osimertinib)
Effective against EGFR T790M resistance mutation bettered CNS penetration, effective in treating brain metastasis
Advanced safety profile compared to previous- generation EGFR TKIs
Once- diurnal oral dosing, which improves patient compliance
Storage and Handling
Keep at room temperature, 20 °C to 25 °C( 68 °F to 77 °F).
Store in tight holders defended from humidity and light.
Keep out of reach of children.
Conclusion
Osicent 80 mg (Osimertinib) is a significant step in the treatment of EGFR- mutantnon-small cell lung cancer. With its veritably high particularity for mutant EGFR, potent clinical effectiveness, CNS penetration, and respectable safety profile, it provides new stopgap for lung cancer cases facing advanced or resistant stages of the complaint. Monitoring is to be kept near, as with all targeted medicines, and rules must be rigorously followed in order to maximize benefits and minimize pitfalls. Consult with an oncologist to determine if Osimertinib should be the first- line medicine grounded on the individual inheritable profile and complaint characteristics of the case.






Reviews
There are no reviews yet.