Don't miss our holiday offer - up to 50% OFF!
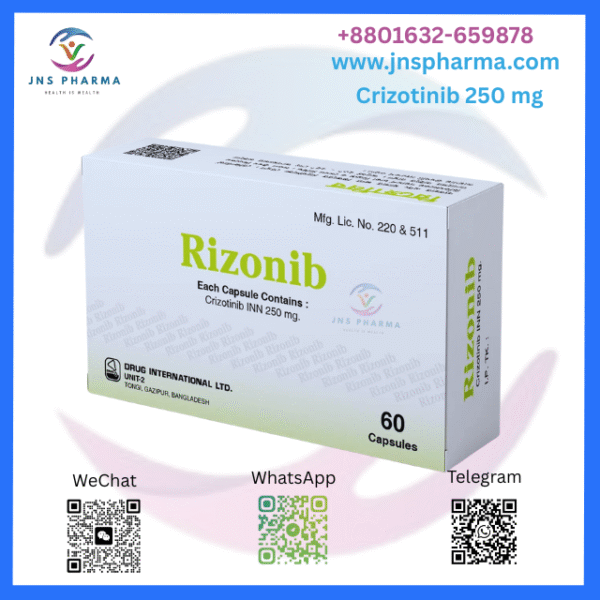
Rizonib 250 mg (Crizotinib)
Rizonib 250 mg, active pharmaceutical component being Crizotinib, is an oral targeted remedy employed in the treatment of certain types of lung cancer. It belongs to the order of tyrosine kinase impediments (TKIs) and is designed to block certain defective proteins that detector cancer cell growth. Rizonib is particularly for cases withnon-small cell lung cancer (NSCLC) with inheritable mutations similar as ALK-positive (anaplastic carcinoma kinase) or ROS1-positive mutations.
Through inhibiting these proteins, Rizonib prevents or slows the cancer cell unbridled proliferation. It’s an important advance in individualized cancer remedy, offering cases a more effective and targeted medicine than conventional chemotherapy.
Composition and Formulation
Active half Crizotinib
Strength 250 mg
Lozenge form Capsule for oral use
Pharmacological class Tyrosine kinase asset (TKI)
Mechanism of Action
Rizonib( Crizotinib) inhibits the exertion of ALK (anaplastic carcinoma kinase) and ROS1 proteins widely, which can come abnormally actuated by inheritable rearrangements or mutations in cancer cells.
In ALK-positive NSCLC, abnormal emulsion between the ALK gene leads to constant activation of cell growth and survival signaling pathways.
In ROS1-positive NSCLC, rearrangements in the ROS1 gene have a analogous effect, leading to cancer progression.
By suppressing these aberrant signals, Rizonib inhibits proliferation in cancer cells, initiates apoptosis (cell death), and helps reduce the excrescence size.
Medical Uses of Rizonib 250 mg
1. Non-Small Cell Lung Cancer (NSCLC)
Rizonib is generally indicated for the treatment of ALK-positive or ROS1-positive locally advanced or metastatic NSCLC cases. inheritable webbing should be done before tradition, as the medicine only works in the case of these mutations.
2. Other Investigational Uses
Outside of lung cancer, Crizotinib is being explored for implicit use in other cancers that are driven by ALK or ROS1 differences, like certain tubercles and nonage cancers, though these uses are n’t yet standard.
Dosage and Administration
Recommended cure 250 mg twice a day (total diurnal dose 500 mg).
Route Oral, with or without food.
Duration: Permanently discontinue upon substantiation of complaint progression or inferior toxin.
Important Administration Instructions
Swallow whole capsules with water, don’t crush or bite.
Take the boluses around the same times each day.
Forget a missed dose if it has been lower than 6 hours to the coming dose — don’t double up.
Curative adaptation is needed for cases with side effects or liver/ order dysfunction.
Side Effects of Rizonib 250 mg
Like with all targetedanti-cancer medicines, Rizonib can beget side effects, which can vary in intensity.
Common Side Effects
Nausea, puking, or diarrhea
Constipation
Fatigue or weakness
lump of hands, ankles, or bases (edema)
Vision changes (blurred vision, flashing lights)
Loss of appetite and weight loss
Severe Side Effects
Liver damage – Increased liver enzymes, hostility, dark- colored urine
Cardiac problems – Bradycardia (slow heart rate), QT extension (abnormal twinkle)
Lung damage – Interstitial lung complaint or pneumonitis
Severe gastrointestinal conditions – Vomiting constantly, stomach cramping
Neurologic effects– Dizziness, neuropathy, or difficulty with collaboration
Cases should report unusual or patient symptoms to their doctor at formerly.
Precautions and Warnings
Inheritable Testing needed
Rizonib should be administered only in ALK-positive or ROS1-positive NSCLC cases. inheritable testing must corroborate the mutation previous to remedy.
Liver Function Monitoring
Routine liver function tests must be performed since Crizotinib can beget hepatotoxicity.
Cardiac Monitoring
Beginning heart complaint cases bear close monitoring of heart rate and ECG.
Eye Health
Visual impairment is common; regular eye examination may be necessary.
Gestation and Breastfeeding
Rizonib is teratogenic. Women with travail eventuality should use effective contraception during remedy and for at least 45 days following the termination of remedy. Breastfeeding is contraindicated during and a many days following remedy.
Medicine relations
Crizotinib is metabolized by the liver enzyme CYP3A4. attendant use with potent CYP3A impediments (e.g., ketoconazole, clarithromycin) or corrupters ( e.g., rifampin, carbamazepine) may be associated with differences in medicine attention. Advise your doctor of all specifics that are being taken.
Storage and Handling
Store at room temperature (15 – 30 °C) in a dry area down from direct light.
Keep out of children’s reach.
Don’t use if package is damaged or expired.
Benefits of Rizonib 250 mg
Targeted remedy specifically targets the shifted ALK/ ROS1 cancer cells, not affecting the maturity of regular cells.
Advanced response rates Cases witness better excrescence reduction compared to standard chemotherapy.
Effects quality of life. Lower toxin compared to standard treatments, allowing cases to perform diurnal conditioning.
Convenience with oral form. Taken as a lozenge at home, lower sanitarium visits necessary.
Limitations
Beneficial only for cases with ALK-positive or ROS1-positive excrescences.
Resistance may develop over time, taking other treatment.
Side effects, though generally controllable, are at times severe and necessitate pullout.
Conclusion
Rizonib 250 mg (Crizotinib) is an innovative treatment for the case of ALK-positive and ROS1-positivenon-small cell lung cancer. By targeting specific inheritable causes of the cancer, it offers cases bettered treatment issues and bettered quality of life compared to standard chemotherapy. still, constant observation, inheritable testing, and adherence to medical advice are consummate for effective and safe administration.
Rizonib is a giant step in the period of customized cancer treatment, giving cases access to knitter- made treatments according to their excrescence’s unique inheritable point.



Reviews
There are no reviews yet.