Don't miss our holiday offer - up to 50% OFF!
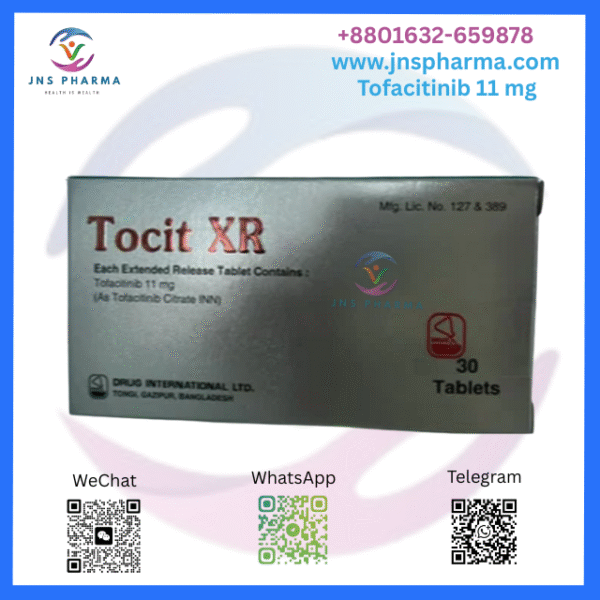
Tocit XR 11 mg (Tofacitinib)
Tocit XR 11 mg is an oral extended- release, formerly- diurnal expression of Tofacitinib, a potent Janus kinase( JAK) asset used to treat several autoimmune conditions. The tablet has been formulated to be administered orally formerly daily, and reduces inflammation and removes symptoms of habitual conditions like rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis. By modulating the vulnerable system, it provides relief from habitual symptoms and improves the case’s quality of life.
Active Ingredient and Mechanism of Action
Tofacitinib, a tiny patch Janus kinase (JAK) asset, and videlicet JAK1 and JAK3 make up the medication’s active ingredient. They’re crucial enzymes in the signaling pathway that executes the seditious signals from cytokine receptors into the cell nexus. Blocking this pathway, Tofacitinib opposes the hyperactive vulnerable response set up in autoimmune conditions.
In discrepancy to conventional biologics that target extracellular pathways, Tofacitinib targets intracellularly and represents an indispensable medium of controlling vulnerable- mediated inflammation. The medium supports oral dosing, a major advantage over injectable remedy.
Indications for Use
The following conditions are treated with Tocit XR 11 mg.
Rheumatoid Arthritis( RA)
For adults with active moderate to severe RA who are reluctant to take methotrexate or other complaint-modifying antirheumatic medications (DMARDs) or who exhibit dogmatism throughout treatment.
Psoriatic Arthritis( PsA)
Reserved for cases with active PsA, particularly when other DMARDs don’t offer acceptable symptom control.
Ulcerative Colitis( UC)
Reserved for use in adult cases with moderate to severe active UC in whom one or further excrescence necrosis factor( TNF) impediments have failed or are unhappy.
Ankylosing Spondylitis( Investigational/ Off- marker in a many homes)
Though not certified in every country, studies are ongoing to determine its efficacity in spondyloarthropathies.
Dosage and Administration
Tocit XR has formerly- diurnal dosing in mind, with the 11 mg tablet furnishing an extended release expression that gives harmonious medicine situations during the day. This minimizes multiple diurnal dosing and improves patient compliance.
Recommended Cure
For utmost suggestions, the cure is 11 mg formerly daily. Exceptions can be made in cases with renal or hepatic impairment, or when given concomitantly with particular remedy( e.g., CYP3A4 asset).
Administration
The tablet should be swallowed whole, not resolve, crushed, or masticated, to save the extended- release parcels.
Benefits of Tocit XR
Accessible Dosing
The extended- release expression improves convenience and makes it easier to cleave to remedy.
Rapid Symptom Relief
Some cases are suitable to have their symptoms resolved within a many weeks of starting remedy.
Non-Biologic Oral Option
In discrepancy to injectable medicines, Tocit XR offers anon-biologic oral option for autoimmune diseases, reducing pain and vexation of injections.
Effective in Refractory Cases
Tocit XR has been set up to be effective in those who failed TNF impediments and other conventional curatives.
Potential Side Effects
As with any medicine, Tocit XR 11 mg may have side effects. Adverse events and serious adverse events are
Common Side Effects
Headache
Upper respiratory infections
Diarrhea
Nasopharyngitis
Serious Side Effects
Increased Infection threat including tuberculosis, herpes zoster, and fungal.
Blood Clots Deep tone thrombosis and pulmonary embolism have passed.
Liver Enzyme Elevations: Liver function monitoring is demanded.
Blood Cell Count Changes including neutropenia and lymphopenia.
malice Slightly increased threat of carcinoma and other malice has been observed with extended remedy.
Blood work needs to be done regularly to cover side effects and find complications beforehand.
Warnings and Precautions
Pre-existing Infections Tocit XR shouldn’t be initiate in cases with an active infection.
Tuberculosis Webbing Cases need to be screen for TB before remedy is started.
Vaccination: During treatment, live immunizations should be avoide. Immunization should be complete previous to remedy inauguration, if doable.
Senior Cases: Greater threat of side effects like infections and cardiovascular events.
Gestation and Lactation: Not to be use in gestation or lactation unless absolutely necessary. Consult with a healthcare professional for threat assessment.
Drug Interactions
Tocit XR is substantially metabolize by CYP3A4 and CYP2C19 enzymes. These enzymes can be by medicines that induce or inhibit them, which can affect the situations of Tofacitinib.
CYP3A4 Impediments (e.g., ketoconazole) can raise Tofacitinib position.
CYP3A4 Corrupters (e.g., rifampin) can reduce medicine effectiveness by dwindling situations of the medicine.
Immunosuppressants attendant use with birth DMARDs or potent immunosuppressants (e.g., azathioprine, cyclosporine) isn’t recommend due to the threat of serious infection.
Storage Instructions
Store at 20 °C to 25 °C( room temperature).
help exposure to light and humidity.
Keep the medicine down from children.
Conclusion
One important medication of option for people with chronic autoimmune diseases such as psoriatic arthritis, ulcerative colitis, and rheumatoid arthritis is Tocit XR 11 mg (Tofacitinib). As an oral formerly diurnal treatment with effective symptom control and a unique medium of action, it’s a precious option as a departure from traditional biologics.
Still, due to the possibility of infections, blood clots, and other serious side effects, it should only be use in discussion with a trained healthcare provider with regular monitoring. The cases should also report unusual symptoms instantly and follow up as per schedule to insure safe and effective treatment.




Reviews
There are no reviews yet.